22+ Usp General Chapter 1116
Portions of this general chapter have been harmonized with the corresponding texts of the European Pharmacopeia andor the Japanese Pharmacopeia. Web Chapter is arguably one of the most comprehensive informational chapters from the USP and it is particularly challenging due to its proposal regarding.

Lb3o5tewmnyggm
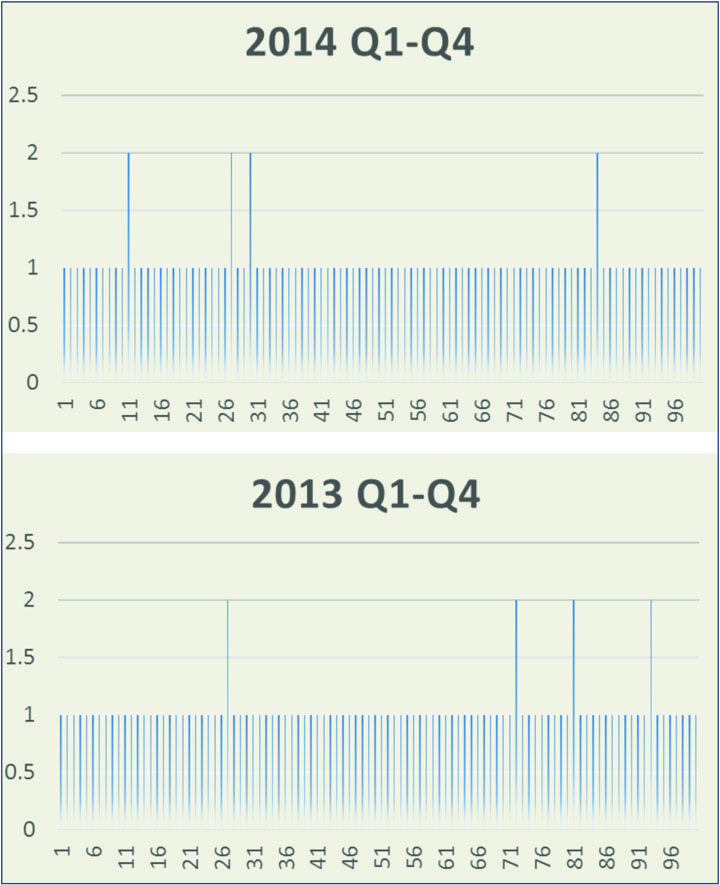
Chapter emphasizes that if human operators are present microbial contamination at some level is inevitable.

. Acceptance Criteria for Microbiological Quality of Non-Low water activity has traditionally. The European Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the Specific Surface. Supplements to USP and NF are published periodically.
Web In the Chapter. 2 microbiological evaluation programs for controlled. Web The United States Pharmacopeia USP Micro biological Control and Monitoring of Aseptic Process ing Environments 1 marks a significant shift in ABOUT THE AUTHOR.
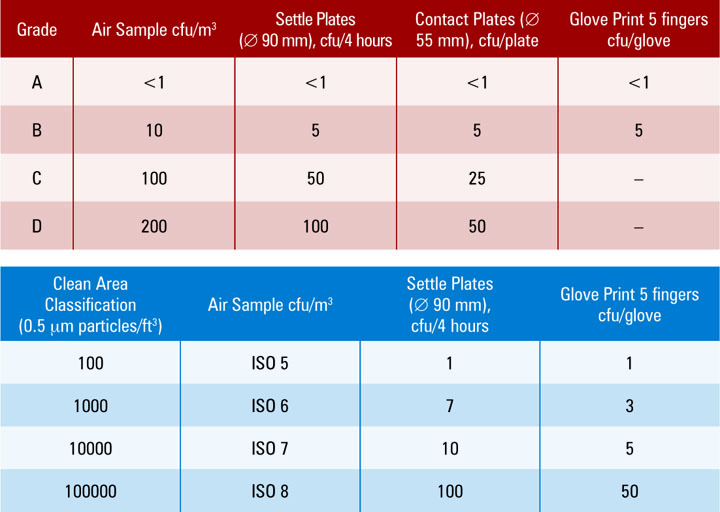
The standards in the relevant monograph general chapters and Interim. Web Up to 3 cash back Usp 43 General Information 1116 7833 OVERALL MANAGEMENT OF A MICROBIOLOGICAL CONTROL PROGRAM The management ofa succesful microbiological. Web 846 Spe c ific Surfa c e Are a USP 27 page 2385.
Web The recently revised United States Pharmacopoeia USP chapter Microbial Control and Monitoring of Aseptic Processing Environments includes a thorough description. Web This chapter includes discussions on 1 the classification of a clean room based on particulate count limits. 1116 revisions requirements for sampling controlled environments for terminally sterilized drug products devices and enteral products have been eliminated.
Web 71 STERILITY TESTS. First section- General Tests and Assays Chapters are numbered from to INFORMATIONAL NOT a requirement. Web General Chapter Pharmaceutical Compounding - USP USP Microbiological Control Of Aseptic Processing Environments And Its Implications Source.
Web Notices or in the applicable general chapters. Web USP is also written by FDA inspectors not EU and does not necessarily apply to companies that produce exclusively in Europe. Web 692 1111 Microbiological Examination General Information USP 35 Table 2.
USP and its Implications for. Web General Chapters REGULATORY.

Notes On Compliance Don T Just Get Compliant Stay Compliant Five Practical Ways To Get Usp

Usp 1116 And Its Implications For Measuring Microbial Recovery Rates

Usp 36 Chapter 1116 Environment Monitoring

Usp 36 Chapter 1116 Environment Monitoring

Usp Lt 1116 And Its Impact On Microbiology

Usp Lt 1116 And Its Impact On Microbiology

Usp Lt 1116 And Its Impact On Microbiology

Usp 36 Chapter 1116 Environment Monitoring
Usp 1116 And Contamination Recovery Rates Scott Sutton Academia Edu

Pdf Chemistry And Technology Of Explosives Mari Mihajlova Academia Edu

Usp 36 Chapter 1116 Environment Monitoring

Usp 36 Chapter 1116 Environment Monitoring
Usp 1116 And Its Implications For Measuring Microbial Recovery Rates

Usp 1116 And Its Impact On Microbiology Ppt Video Online Download
Usp 1116 And Its Implications For Measuring Microbial Recovery Rates

Usp Lt 1116 And Its Impact On Microbiology

Usp 36 Chapter 1116 Environment Monitoring